A new approach towards solving mysteries of the interstellar medium
MBI researchers observe charge migration in hydrocarbons for the first time
It is one of the most intriguing questions in astrochemistry: the mystery of the diffuse interstellar bands (DIBs), a collection of about 400 absorption bands that show up in spectra of light that reaches the earth after having traversed the interstellar medium. Despite intense research efforts over the last few decades, an assignment of the DIBs has remained elusive, although indications exist that they may arise from the presence of large hydrocarbon molecules in interstellar space. Recent experiments at the Max Born Institute lend novel credibility to this hypothesis.
Among the hydrocarbons that are possible carriers of the DIBs, polycyclic aromatic hydrocarbons (PAHs) are considered to be particularly promising. The presence of PAH molecules was previously inferred in many astronomical objects, as well as in the interstellar medium of the Milky Way. However, within the astronomical community, the linewidths of the DIBs, which are indicative of the lifetimes of the excited states that are involved in the absorption process, are often considered as an argument that speaks against the PAHs. The new experiment was performed in collaboration with scientists from the university of Lyon and aided by theoretical input from scientists at the universities of Heidelberg, Hyderabad and Leiden. It has been shown that the lifetimes of excited states of small to medium-size PAHs are consistent with the linewidths that are observed for the DIBs.
In the experiments, a series of small to medium-size PAH molecules (naphthalene, anthracene, pyrene and tetracene, containing 2-4 benzene-like aromatic rings), were ionized by an ultrashort extreme-ultraviolet (XUV) laser pulse. As a result of electron correlation, the absorption of an XUV photon not only led to removal of one of the electrons, but furthermore to electronic excitation of the molecular ion left behind. The lifetimes of these excited cationic electronic states were monitored by probing the ions with a moderately strong, time-delayed infrared (IR) laser pulse. When the ions are formed, the electronic excitation is at its highest, and only one or a few IR photons are needed to remove a second electron. However, a little later, when the ion relaxes and energy is transferred from the electronic to the vibrational degrees of freedom, more IR photons are needed to remove the second electron. In other words, monitoring the formation of doubly-charged ions as a function of the time delay between the XUV and IR laser pulses allowed extraction of the lifetimes of the states formed by the XUV ionization process. As it turned out, and as was further supported by high-level calculations, these lifetimes of a few 10s of femtoseconds are well within the range of what is required for potential carriers of the DIBs.
Beyond the implications for the DIBs, the new experiments have implications for the further development of attosecond science. One of the most sought-after goals in attosecond science at the moment, is the observation of charge migration, i.e. ultrafast (attosecond to few-femtosecond) motion of an electron or hole through a molecular structure. It has been proposed that charge migration may provide new opportunities for control of chemical reactivity, a goal that is as old as the chemical research itself. First indications that attosecond to few-femtosecond time-scale dynamics can be observed in polyatomic molecules were obtained by researchers at the university of Milano last year. The PAH molecules that were investigated in the experiments at MBI represent the largest molecular species yet to which ultrafast XUV-IR pump-probe spectroscopy has been applied. Besides the insights into ultrafast electronic relaxation obtained from the current work, the theoretical work performed in order to interpret the experiments suggests that PAH molecules are also ideal candidates for observing attosecond to few-femtosecond timescale charge migration. Such experiments will therefore be attempted next.
Figure:
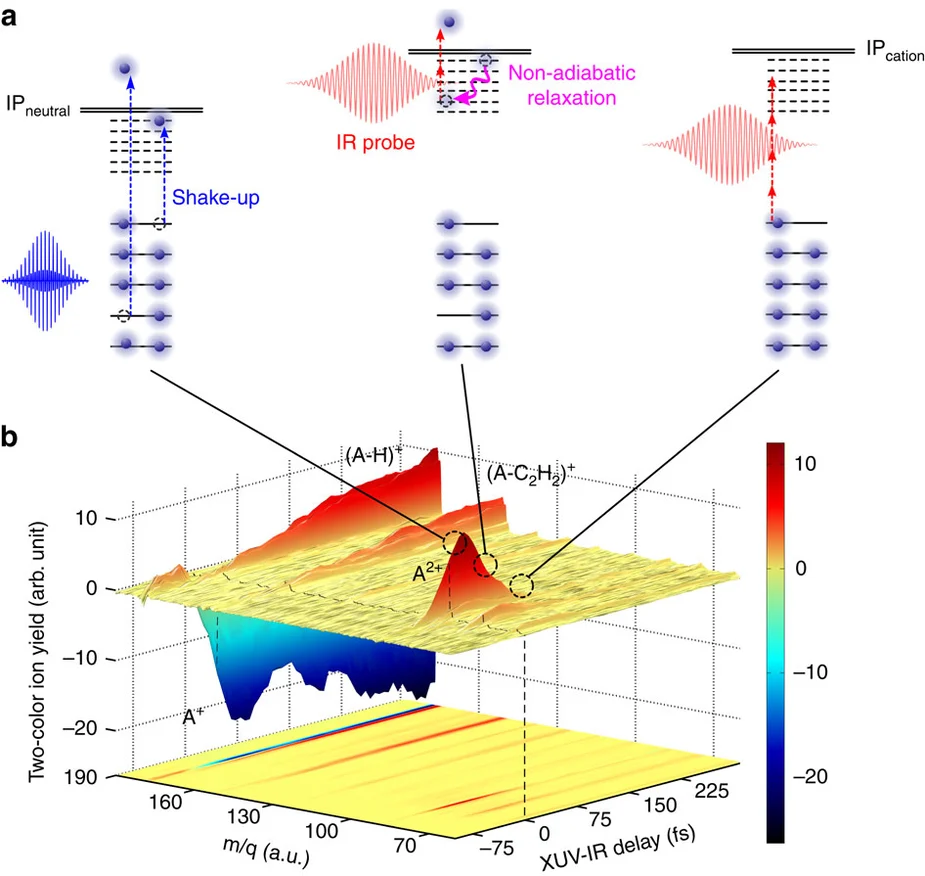
(a) Schematic of the XUV-induced dynamics in PAH molecules studied in this paper. Excited states are created in the valence shell of the cation through one of two possibilities, namely the formation of a single-hole configuration or the formation of a 2hole-1particle configuration (involving a shake-up process) (left) (IP stands for Ionization potential). The cation can be further ionized by the IR probe laser, provided that non-adiabatic relaxation has not taken place yet (middle). After relaxation, the IR probe cannot ionize the cation anymore (right).
(b) Two-colour XUV-IR ion signals measured in the case of anthracene, as a function of the detected mass-to-charge ratio and the XUV-IR delay. XUV-only and IR-only signals have been subtracted. The XUV pump and IR probe pulses overlap at zero delay (black dashed line). A red colour corresponds to a signal increase, while a blue colour signifies depletion. For positive XUV-IR delays, a very fast dynamics is observed for the doubly charged anthracene ion (A2+, m/q=89). As explained in the text, the measurement reflects non-adiabatic relaxation in the anthracene cation (A+). The dynamics observed in the first fragment (A-C2H2+) is not discussed in this article.
Original publication: Nature Communications 6, DOI:10.1038/ncomms8909
XUV excitation followed by ultrafast non-adiabatic relaxation in PAH molecules as a femto-astrochemistry experiment
A. Marciniak, V. Despré, T. Barillot, A. Rouzée, M.C.E. Galbraith, J. Klei, C.-H. Yang, C.T.L. Smeenk, V. Loriot, S. Nagaprasad Reddy, A.G.G.M. Tielens, S. Mahapatra, A. I. Kuleff, M.J.J. Vrakking & F. Lépine
Contact:
Prof. Dr. Marc Vrakking
Director Division A: Attosecond Physics
Max-Born-Institut
Tel.: +49 30 6392-1200
Email: vrakking(at)mbi-berlin.de