Time-resolving the Motion of Electrons in Atoms, Molecules and Solids on their Natural Timescale
Attosecond Science opens new Avenues in Femtochemistry
Attosecond Science is a new exciting frontier in contemporary physics, aimed at time-resolving the motion of electrons in atoms, molecules and solids on their natural timescale. Electronic dynamics derives from the creation and evolution of coherence between different electronic states and proceeds on sub-femtosecond timescales. In contrast, chemical dynamics involves position changes of atomic centers and functional groups and typically proceeds on a slower, femtosecond timescale inherent to nuclear motion.
Nonetheless, there are exciting ways in which chemistry can hugely benefit from the technological developments pushed forward in the vibrant field of Attosecond Science. This was exploited in the work recently published by Lorenz Drescher and coworkers. Attosecond pulses are generated in the process of High Harmonic Generation (HHG), in which infrared photons are upconverted to the extreme ultraviolet (XUV) frequency domain in a highly non-linear interaction of intense coherent light and matter. The short duration of attosecond pulses implies a frequency spectrum with photon energies spanning from a few electron volts (eV) to hundreds of eV. Such broad and continuous frequency spectra are ideally suited for core shell absorption measurements in molecules.
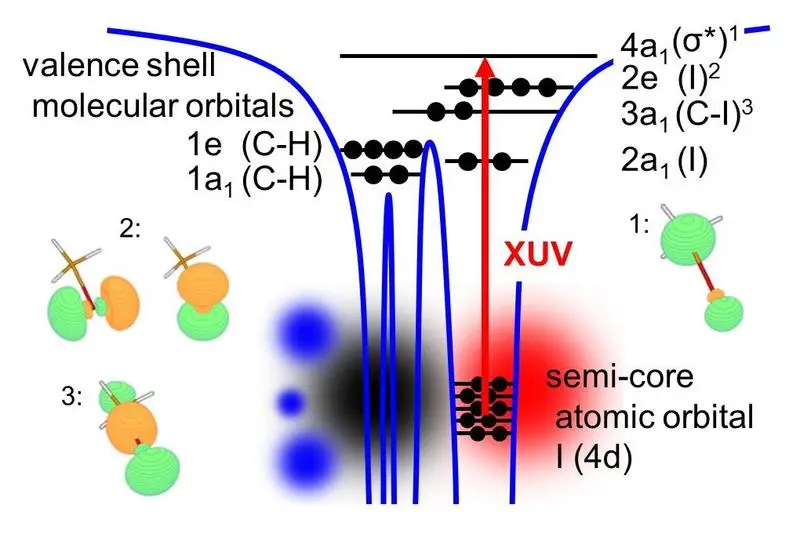
Core shell to valence shell transitions are a unique probe of molecular structure and dynamics. Core-to-valence transitions are element specific, due to the highly localized nature of core orbitals on specific atoms. On the other hand the intramolecular local environment of specific atomic sites is encoded, since an electron is lifted from a core orbital to a hole in the valence shell, affected by chemical bonding (see Fig. 1). Importantly, these transitions typically correspond to very short lifetimes of only a few femtoseconds. The use of ultrashort XUV pulses hence gives a new twist to the ultrafast studies of chemistry: It allows to probe chemical dynamics, initiated by a UV pump laser pulse, from the perspective of different reporter atoms within a molecule in an XUV transient absorption experiment. This is now beginning to be explored by a number of groups around the world.
In the experiment carried out by Drescher and coworkers at the MBI, photodissociation of iodomethane (CH3I) and iodobenzene (C6H5I) was studied with time-resolved XUV transient absorption spectroscopy at the iodine pre-N4,5 edge, using femtosecond UV pump pulses and XUV probe pulses from HHG (see Fig.2). For both molecules the molecular core-to-valence absorption lines were found to fade immediately, within the pump-probe time-resolution. Absorption lines converging to the atomic iodine product however emerge promptly in CH3I but are time-delayed in C6H5I. In CH3I, we interpret this observation as the creation of an instantaneous new target state for XUV absorption by the UV pump pulse, which is then subject to relaxation of the excited valence shell as the molecule dissociates. This relaxation shows in a continuous shift in energy of the emerging atomic absorption lines in CH3I, which we measured in the experiment. In contrast, the delayed appearance of the absorption lines in C6H5I is indicative of a UV created vacancy, which within the molecule is initially spatially distant from the iodine reporter atom and has to first travel intramolecular before being observed. This behaviour is attributed to the dominant π → σ* UV excitation in iodobenzene, which involves the π orbital of the phenyl moiety.
While in the current work only a simplistic independent particle model was used to rationalize the observed experimental findings, MBI with its newly created theory department provides unique opportunities for joint experimental and theory studies on XUV transient absorption of photochemical processes. This will involve a new theoretical approach developed recently by researchers from MBI together with colleagues in Canada, the UK and Switzerland, which was recently submitted as a publication.
Original Publication: Journal of Chemical Physics Communication, 145, 011101 (2016)
XUV transient absorption spectroscopy of iodomethane and iodobenzene photodissociation
L. Drescher, M.C.E. Galbraith, G. Reitsma, J. Dura, N. Zhavoronov, S. Patchkovskii, M.J.J. Vrakking, and J. Mikosch
Contact
Max-Born-Institut für Nichtlineare Optik und Kurzzeitspektroskopie (MBI)
Max-Born-Str. 2A , 12489 Berlin
Dr. Jochen Mikosch
Tel. +49 (0) 30 6392 1240
mikosch(at)mbi-berlin.de
www.mbi-berlin.de