Insights into photoacid electronic structure
Using ultrafast X-ray spectroscopy, the electronic charge distributions of photoacids could be studied at BESSY II
Photoacids are molecules that release a proton upon electronic excitation, thus enhancing the acidity of a liquid. Pioneering work by Theodor Förster has shown the direct relationship between the wavelength position of optical absorption and acidity properties with which the increase in acidity in the first electronic excited state can be quantified. However, underlying full microscopic explanations for the photoacidity phenomenon have remained sparse.
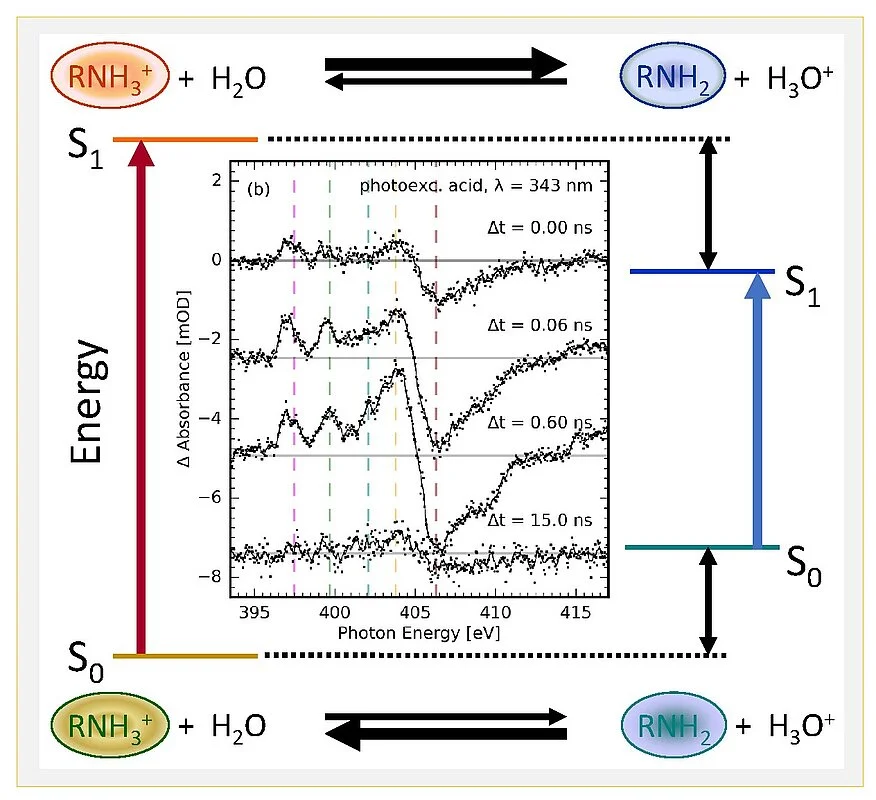
With ultrafast X-ray spectroscopy, locally probing the electronic structure of a proton donating group of an amine aromatic photoacid has now provided direct insight in the changes of electronic structure. The long standing open question for photoacidity has now finally been resolved: major electronic structure changes occur on the base side of the so-called Förster cycle, whereas the acid side plays a minor role.
Photoacids have been known for more than 70 years. Theodor Förster has been the first to correctly describe the observations of absorption and fluorescence spectra of photoacids, and connect positions of the electronic transitions giving rise to optical absorption bands to the increased acidity properties of photoacids in the electronic excited state. Many research activities have been pursued in the following decades, but apart from quantum chemical calculations of photoacid molecules of medium size, focussing on the intramolecular electronic charge distribution changes of the proton donating moieties of photoacids, microscopic insight have remained limited. Some of these studies have indicated – in line with previous suggestions based on physical organic principles – that the effects of electronic excitation are much more pronounced on the conjugate photobase side of the Förster cycle (Fig. 1).
Scientists from the Max Born Institute in Berlin, Stockholm University, the University of Hamburg, Helmholtz-Zentrum Berlin, Ben-Gurion University of the Negev in Beersheva and Uppsala University, have now successfully pursued a novel combined experimental and theoretical approach to study the electronic charge distributions of photoacids along the four stages of photoacids provide direct microscopic insight into the electronic structural changes of the proton donating amine group of an aminopyrene derivative in aqueous solution.
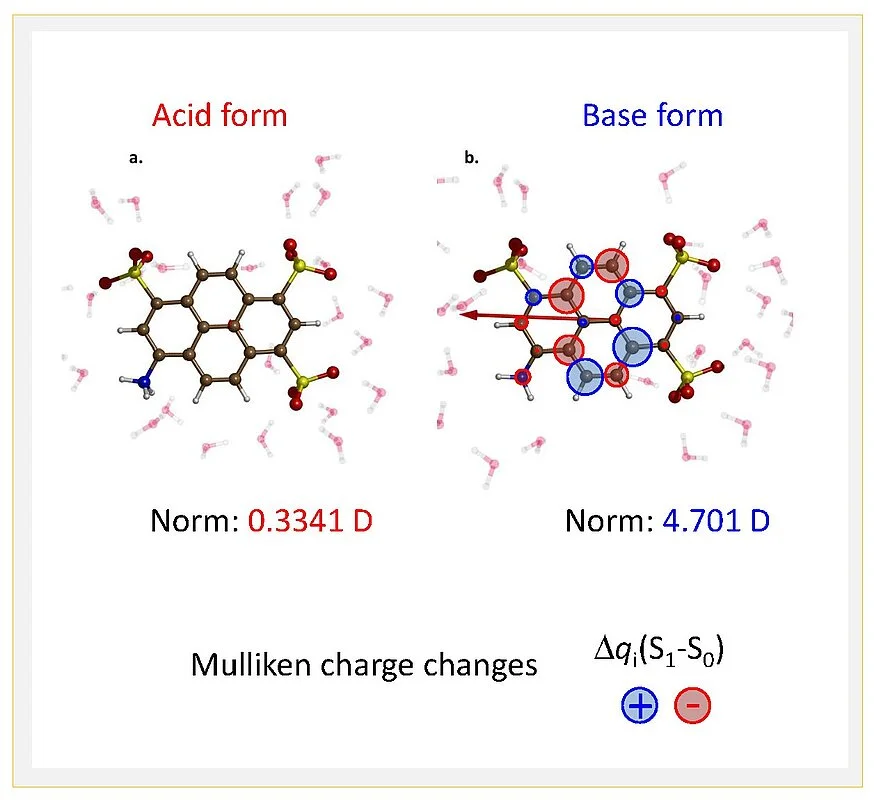
The K-edge X-ray absorption spectra of nitrogen atoms in the molecular structure were measured at the synchrotron BESSY II in transmission mode to locally probe electronic structure on ultrafast time scales. Together with quantum chemical calculations, such results provide a consistent picture of photoacid behaviour (Fig. 2): electronic charge distributions of the proton donating group are only minor on the photoacid side, but substantial on the conjugate photobase side. Yet the overall dipole moment change of the whole molecule is as important as the local charge distribution changes, hence solvation dynamics by the solvent water is the second important factor governing photoacidity.
Publication
Electronic Structure Changes of an Aromatic Amine Photoacid along the Förster Cycle
Sebastian Eckert, Marc-Oliver Winghart, Carlo Kleine, Ambar Banerjee, Maria Ekimova, Jan Ludwig, Jessica Harich, Mattis Fondell, Rolf Mitzner, Ehud Pines, Nils Huse, Philippe Wernet, Michael Odelius, and Erik T. J. Nibbering
Angew. Chem. Int. Ed. 2022, e202200709. URL, DOI oder PDF
Contact:
Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy
Nonlinear Processes in Condensed Matter
Dr. Marc-Oliver Winghart
Phone +49 30 6392-1496
Email marc-oliver.winghart(at)mbi-berlin.de
Dr. Erik Nibbering
Phone +49 30 6392-1477
Email erik.nibbering(at)mbi-berlin.de
Press release MBI, 25 March 2022