Magnesium ions slow down water dynamics on short length scales
Experiments by MBI, FU Berlin and LMU Munich show the influence of ions on the dynamics of surrounding water molecules
The presence of ions affects the structure and dynamics of water on a multitude of length and time scales. New results from ultrafast spectroscopy and theoretical analyses show that the water environment of specific pairs of magnesium and sulfate ions has a decisive impact on the dynamics of the aqueous solutions.
Liquid water, the native medium for biochemical and cellular processes, consists of a complex network of polar molecules connected by hydrogen bonds. Water responds to the presence of a solute by changing its local structure. The influence of negatively and positively charged ions on liquid water is usually classified via the Hofmeister series which ranks ions based on their ability to structure the water around them or to disrupt the water structure. The microscopic origin and molecular mechanisms of the Hoffmeister series are controversial, despite many years of research. However, the Hoffmeister series has great relevance because it characterizes the influence ions exert on biomolecules dissolved in water.
Recent experiments and simulations have now revealed a significantly more complex influence of ions on the dynamics of surrounding water molecules. The study, published in the journal ACS Physical Chemistry Au, combines spectroscopic experiments with in-depth theoretical analyses of the molecular interactions and dynamics. The researchers from the Max Born Institute in Berlin, Freie Universität Berlin and the Ludwig-Maximilian-University in Munich used the asymmetric stretching vibrations of sulfate (SO42-) ions as locally sensitive probes to map the dynamic properties of the environment. In this context, hydrated sulfate ions are a prototypical model system, as they are commonly found in minerals and are of great importance in physiology and biochemistry. In order to obtain information about the local hydration environment, the researchers used the experimental technique of two-dimensional infrared spectroscopy in the femtosecond time domain and compared the behavior of sulfate ions in the presence of sodium (Na+) or magnesium (Mg2+) ions at low ion concentrations (Figure 1). They find that the presence of Mg2+ ions reduces the ultrafast fluctuations of the water shell around a sulfate ion, leading to a specific slowdown in the solvation dynamics of hydrated MgSO4 compared to aqueous Na2SO4 solutions.
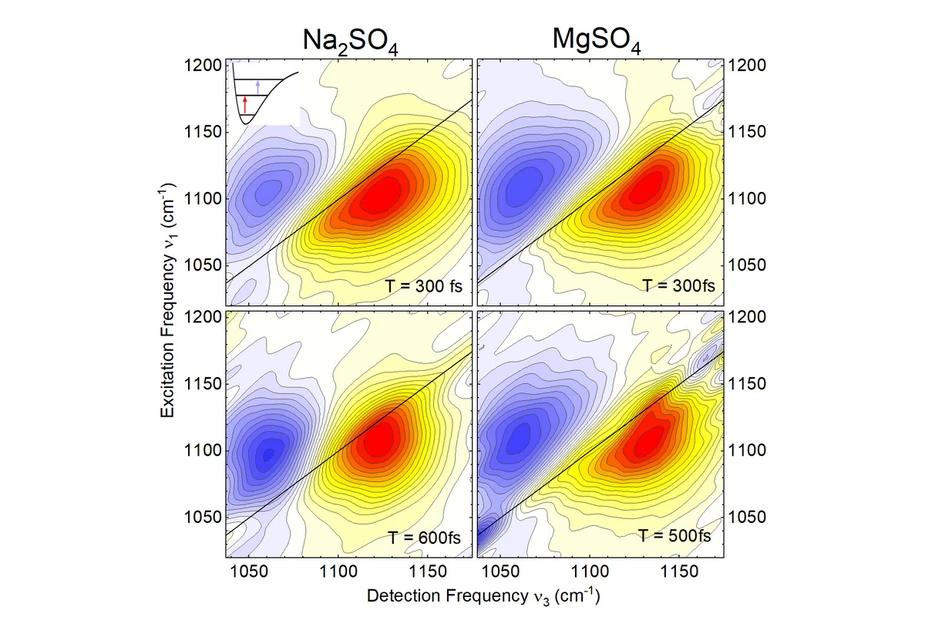
Figure 1: Two-dimensional infrared spectra of the asymmetric stretching vibration of the SO42- ion for sodium sulfate (Na2SO4, left column) and magnesium sulfate (MgSO4, right column) in water. The strength of the vibrational excitation is plotted as a function of the excitation frequency ν1 and the detection frequency ν3. Yellow-red contours correspond to excitations of the fundamental vibrational transition between the v=0 and 1 states of the vibration (red arrow in the inset image at top left), while blue contours are due to overtone excitations (v=1 to 2, blue arrow). For sodium sulfate, a change in the red-yellow contour from an elliptical line shape (top left) to a more round shape (bottom left) is observed within about 300 fs. This is due to rapidly fluctuating forces exerted on the sulfate ion by the water environment. In contrast, for magnesium sulfate (right column) the elliptical line shape is preserved on this time scale. Here, fast fluctuations are largely suppressed by the presence of magnesium ions in the immediate vicinity of the sulfate ion.
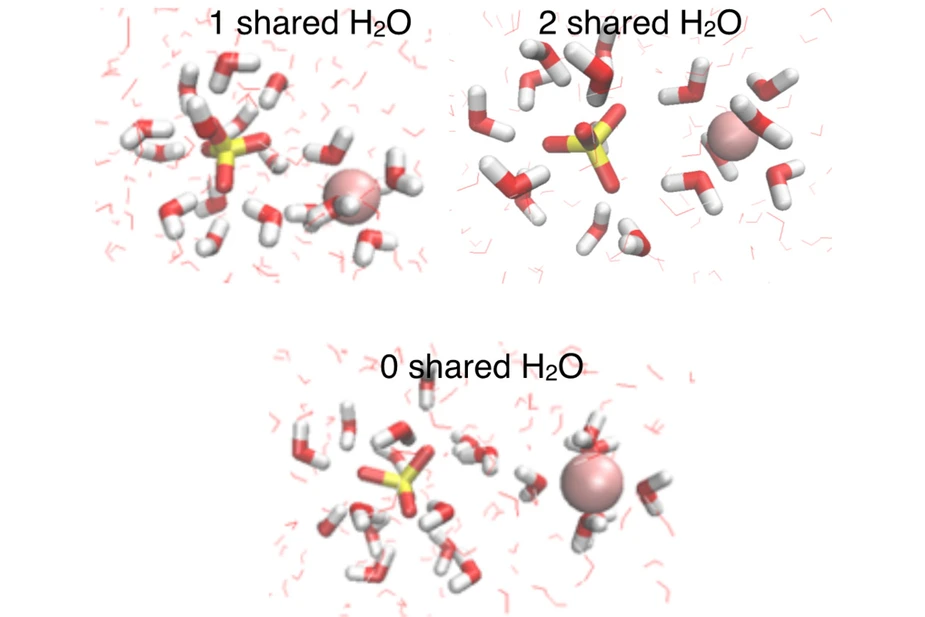
Extensive simulations provide a microscopic view of the observed dynamics and reveal a molecular picture in which the slowdown of the water dynamics arises from structural features of SO42- - Mg2+ ion pairs that share water molecules in their hydration shells (Figure 2). Contrary to the widespread account in the literature, the described effects are of short range and limited to the first 1-2 water layers around the sulfate ion. In contrast to the Hoffmeister series, which classifies the effect of ion types on the water structure, the new results demonstrate a particular relevance of individual solvation geometries of specific ion pairs for the dynamics of dilute aqueous systems.
Figure 2: Structural representation of Mg2+- SO42- ion pairs in aqueous solution. The simulations yield a molecular picture in which the slowdown of hydration dynamics results from structural features of SO42-- Mg2+ ion pairs that share 1 or 2 water molecules in their hydration shells (top), whereas ion pairs with separate, complete hydration shells (bottom) have little effect on water dynamics. Sulfur atoms are shown in yellow, magnesium atoms in pink, oxygen atoms in red and hydrogen atoms in white; water molecules in the first hydration shells around the ions are highlighted in bold representation.
Publication
Short-Range Cooperative Slow-down of Water Solvation Dynamics Around SO42−−Mg2+ Ion Pairs.
Achintya Kundu, Shavkat I. Mamatkulov, Florian N. Brünig, Douwe Jan Bonthuis, Roland R. Netz, Thomas Elsaesser, and Benjamin P. Fingerhut
ACS Physical Chemistry Au (open access, ACS Editor’s Choice) (2022), doi: 10.1021/acsphyschemau.2c00034, https://pubs.acs.org/doi/full/10.1021/acsphyschemau.2c00034
Contact
Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy
Dr. Achintya Kundu
Phone +49 30 6392-1414
Email Achintya.Kundu(at)mbi-berlin.de
Prof. Dr. Thomas Elsässer
Phone +49 30 6392-1400
Email Thomas.Elsaesser(at)mbi-berlin.de
Forschungsverbund Berlin e.V.
Anja Wirsing
Press Office
Phone +49 30 6392-3337
wirsing(at)fv-berlin.de
Press release MBI, 12 October 2022