Molecular orbitals determine stability
Team at HZB has elucidated the influence of the electronic structure on the stability of fumarate, maleate and succinate dianions
Carboxylic acid dianions (fumarate, maleate and succinate) play a role in coordination chemistry and to some extent also in the biochemistry of body cells. An HZB team at BESSY II has now analysed their electronic structures using RIXS in combination with DFT simulations. The results provide information not only on electronic structures but also on the relative stability of these molecules which can influence an industry's choice of carboxylate dianions, optimizing both the stability and geometry of coordination polymers.
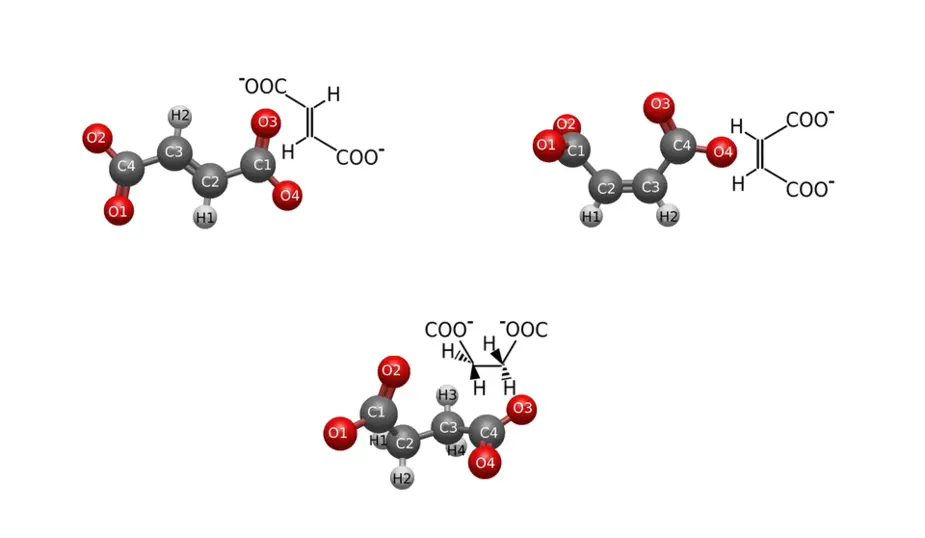
Carboxylic acid dianions of the type C4H2O4 or C4H4O4 (fumarate, maleate and succinate) can have different geometries (cis or trans) and different properties. Some variants are key in coordination chemistry, incorporating metallic elements into organic compounds, other variants play a role in biological processes. Fumarate and succinate, for example, are formed as intermediate products in the mitochondria of cells. Maleate, on the other hand, which is usually not formed in natural processes, is used in industrial applications that require durable materials. For environmental reasons, however, the question arises as to whether these compounds last forever or are biodegradable.
The stability of fumarate, maleate and succinate dianions is not only influenced by their molecular geometries, but also by the electronic structure of the molecules, in particular the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). However, the influence of the molecular orbitals on stability of these molecules has not been researched.
RIXS and XAS at BESSY II
Now, a team at HZB led by Prof. Alexander Föhlisch has elucidated the influence of the electronic structure on the stability of fumarate, maleate and succinate dianions. “We analysed these compounds at BESSY II with two different, very powerful methods,” says Dr Viktoriia Savchenko, first author of the study. X-ray absorption spectroscopy (XAS) can be used to investigate the unoccupied electronic states of a system, while resonant inelastic X-ray scattering (RIXS) provides information about the occupied highest orbitals and about interactions between the HOMO-LUMO orbitals. The results can be related to macroscopic properties, especially stability.
Maleate potentially less stable
The analysis of the spectral data shows that maleate is potentially less stable than fumarate and succinate. What’s more: The analysis also explains why: The electronic density in the HOMO orbital at the C=C bond between carboxylate groups could lead to weaker binding of maleate with molecules or ions. Fumarate and succinate, on the other hand, could be more stable, as their HOMO orbitals are equally delocalised.
“This means that there is a chance that maleate could be degraded by certain substances,” says Savchenko.
Publication:
Physical Chemistry Chemical Physics (2024): Electronic structure, bonding and stability of fumarate, maleate, and succinate dianions from X-ray spectroscopy
Viktoriia Savchenko, Sebastian Eckert, Mattis Fondell, Rolf Mitzner, Vincius Vaz da Cruz and Alexander Föhlisch
DOI: 10.1039/D3CP04348G
Contact:
Helmholtz-Zentrum für Materialien und Energie
Institute Methods and Instrumentation for Synchrotron Radiation Research
Viktoriia Savchenko
(030) 8062-14887 / -14987
viktoriia.savchenko(at)helmholtz-berlin.de
Dr. Antonia Rötger
Press Officer
(030) 8062-43733
antonia.roetger(at)helmholtz-berlin.de
Press release HZB, 7 February 2024