A cage structured material transforms into a performant catalyst for producing green hydrogen
The promising material class of clathrates was investigated at BESSY II
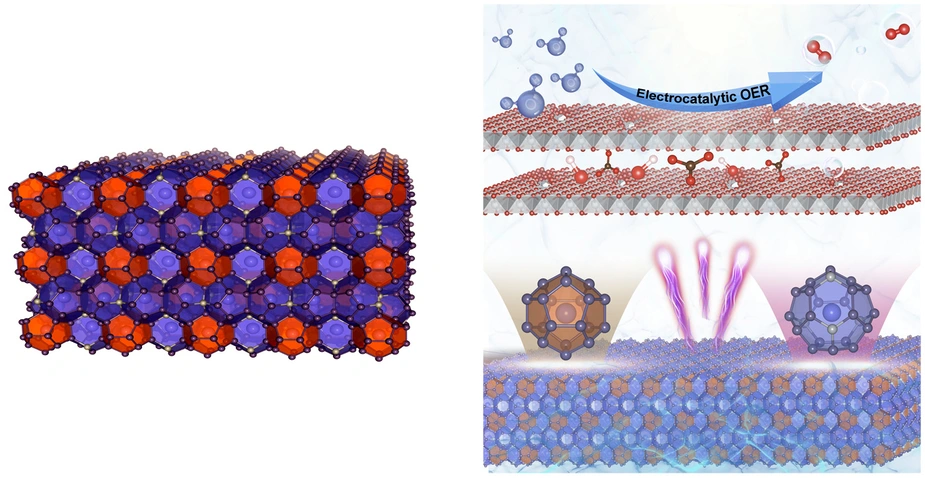
Clathrates are characterised by a complex cage structure that provides space for guest ions too. Now, for the first time, a team has investigated the suitability of clathrates as catalysts for electrolytic hydrogen production with impressive results: the clathrate sample was even more efficient and robust than currently used nickel-based catalysts. They also found a reason for this enhanced performance. Measurements at BESSY II showed that the clathrates undergo structural changes during the catalytic reaction: the three-dimensional cage structure decays into ultra-thin nanosheets that allow maximum contact with active catalytic centres. The study has been published in the journal ‘Angewandte Chemie’.
Hydrogen can be produced by electrolysis of water. If the electrical energy required for this process comes from renewable sources, this hydrogen is even carbon neutral. This 'green' hydrogen is seen as an important building block for the energy system of the future and is also needed in large quantities as a raw material for the chemical industry. Two reactions are crucial in electrolysis: hydrogen evolution at the cathode and oxygen evolution at the anode (OER). However, the oxygen evolution reaction in particular slows down the desired process. To speed up hydrogen production, more efficient and robust catalysts for the OER process need to be developed.
Clathrates, a structure build of cages
Currently, nickel-based compounds are considered to be good and inexpensive catalysts for the alkaline oxygen evolution reaction. This is where Dr. Prashanth Menezes and his team come in. ‘The contact between the active nickel centres and the electrolyte plays a crucial role in the efficiency of a catalyst,' says the chemist. In conventional nickel compounds, this surface area is limited. ‘We therefore wanted to test whether nickel-containing samples from the fascinating class of materials known as clathrates could be used as catalysts'.
The materials are made of Ba8Ni6Ge40 and were produced at the Technical University of Munich. Like all clathrates, they are characterised by a complex crystalline structure of polyhedral cages, in this case, formed by germanium and nickel, enclosing barium. This structure gives clathrates special properties that make them interesting as thermoelectrics, superconductors or battery electrodes. However, until now, no research group had considered of investigating clathrates as electrocatalysts.
Experiments at universities and BESSY II
The electrochemical measurements showed that the Ba₈Ni₆Ge₄₀ as a catalyst exceeded the efficiency of nickel based catalysts at a current density of 550 mA cm⁻², a value also used in industrial electrolysis. The stability was also remarkably high: even after 10 days of continuous operation, the activity did not decrease significantly.
The team used a combination of experiments to find out why the material is so remarkably well suited. At BESSY II, they studied the samples using in situ X-ray absorption spectroscopy (XAS), while basic structural characterisation were carried out at the Freie and Technische Universität Berlin.
From cage to sponge
Their analysis showed that the Ba8Ni6Ge40 particles in the aqueous electrolyte undergo a structural transformation under an electric field: germanium and barium atoms dissolve out of the former three-dimensional framework. ‘The germanium and barium atoms make up almost 90 % of the clathrate starting material and they are completely washed out, leaving behind highly porous, sponge-like nanolayers of the remaining 10 % nickel that offer a maximum surface area,‘ says Dr. Niklas Hausmann from Menezes’ team. This transformation brings more and more catalytically active nickel centres into contact with the electrolyte.
‘We were actually surprised by how well these samples work as OER catalysts. We expect that we can observe similar results with other transition metal clathrates and that we have discovered a very interesting class of materials for electrocatalysts,’ says Menezes.
Publication:
Angewandte Chemie (2025): Ba-Ni-Ge Clathrate Transformation Maximizes Active Site Utilization of Nickel for Enhanced Oxygen Evolution Performance
Ziliang Chen, Hongyuan Yang, J. Niklas Hausmann, Stefan Mebs, Viktor Hlukhyy, Holger Dau, Matthias Driess, Prashanth W. Menezes
DOI: 10.1002/anie.202424743
Contact:
Helmholtz-Zentrum Berlin für Materialien und Energie
Department Materials Chemistry for Catalysis
Jan Niklas Hausmann
+49 30 8062-12957
niklas.hausmann(at)helmholtz-berlin.de
Dr. Prashanth Menezes
+49 30 8062-14630
prashanth.menezes(at)helmholtz-berlin.de
Dr. Antonia Rötger
Press Officer
+49 30 8062-43733
antonia.roetger(at)helmholtz-berlin.de
Press release HZB, 17 April 2025